How HP Is 3D Printing Critical Medical Supplies
The quick response to the global pandemic is helping meet the urgent needs of medical personnel around the globe.
By Jennifer Ceaser
As the COVID-19 virus spread around the globe and severely impacted the global-supply chain of vital medical equipment for healthcare professionals, HP’s 3D manufacturing lab in Barcelona, Spain started getting urgent requests from the medical community.
On March 23, Juan Angel Muñoz López, a nursing supervisor at Príncipe de Asturias Hospital in Alcalá de Henares, outside of Madrid, the epicenter of Spain’s coronavirus pandemic, sent an email to Barcelona’s 3D Printing and Digital Manufacturing Center of Excellence. The email made its way to Clara Remacha Corbalán, an advanced technical consultant and HP’s applications lead to COVID-19 response who recalls, “[Muñoz] told us they had already reached the point where they have to decide who lives and who doesn’t; they don’t have enough respirators for everyone.” The hospital’s ICU nursing staff had designed a part that could connect multiple standard elements to create a CPAP device, which can help increase patients’ blood oxygen levels above 90%, avoiding the need for a ventilator. But they had no way to produce it.
The HP team recognized the need to act fast. “Within two days [of receiving the message] we had evaluated the design, printed them, and sent them to the hospital for validation,” says Remacha. “We had a dedicated person calling the agency [Agencia Española de Medicamentos y Productos Sanitarios, or AEMPS, similar to the FDA in the US] every morning and every afternoon asking what additional documentation was needed.” At the same time, they were meeting with the Spanish government every two or three days to push for approval of the device.
Within two weeks the CPAP Cross-connector was approved for use in Spanish hospitals; the device has been in use since April 6 with 500 connectors having been produced on 3D printers in the Barcelona plants, which has the capacity to produce 10,000 per week.
“The hospital is the battlefield, and the healthcare workers are the soldiers. Without the help of HP, in projects like this CPAP connector, we would not have enough weapons to beat this nightmare,” says Muñoz.
Rapid response to the crisis
Industries, from beauty to fashion to automotive, have rushed to help over the past two months, repurposing their production facilities and R&D capabilities, manufacturing mass quantities of hand sanitizer, producing tens of thousands of masks and gowns, and retooling factories to assemble face shields.
“Across HP, there has been a huge commitment to help in the COVID-19 battle, and the organization has gone above and beyond to transform how we design and quickly scale up production for critical medical items,” says Fabio Annunziata, head of strategy and planning for HP’s 3D Printing & Digital Manufacturing business, and lead of HP’s 3D Printing COVID-19 taskforce.
The 3D printing industry has also stepped up to design and manufacture critical parts to help meet the urgent need, producing personal protective equipment — including masks and face shields — for medical personnel on the front lines of battling COVID-19. HP’s deep expertise in the industry means that it was able to take immediate action, and R&D centers in Barcelona, and Oregon, Washington, and California, are coordinating with government agencies and health experts around the globe to identify the parts most in need, validate designs, and begin production. “We already had a market development team with strong connections in the industry, dedicated to exploring the use of 3D printing in the medical space, including medical equipment, orthotics, and many other applications,” says Remacha. Since announcing its efforts March 24, HP and its global community of partners and customers has produced more than 150,000 parts for medical applications and growing.
On its website, HP and its partners have made 3D printable designs freely available with applications for medical usage including face shields, masks, mask adjusters, wrist covers (which cover exposed skin), and hands-free door openers. The page is continually updated as designs are validated. Hospitals can also submit requests for products. This has been a crucial way in which HP has been able to connect with the medical community to create solutions for healthcare workers.
“We received hundreds of requests for face shields on the website, but it was important to evaluate and test the designs to come up with ones that really worked,” says Remacha. “We decided in addition to delivering face shields to hospitals, we would also pursue full certification of the device based on the European standard, EN166, which was received [the week of April 13].”
The face shields were considered a priority for the team, as Spain has the world’s highest reported rate of COVID-19 infection for doctors and nurses. Remacha and the R&D team consulted with a Barcelona doctor on the design, which included modifying it to have a more comfortable headband. Another team ensured that the device would meet the standards for regulation in Spain. A third team handled the supply chain side (while the 3D printed part is the headwear, the shields are sourced elsewhere). Within two days the first version had been produced and sent to the hospital for feedback.
“On a Saturday morning [March 21], we knew nothing about face shields, by Monday morning they were already at the hospital,” says Remacha. After some design tweaks, by Wednesday, the third version was finalized, which is one of three face shield designs on the site available for download. To date, some 8,000 face shields have been produced at the Barcelona facilities.
A shortage of swabs
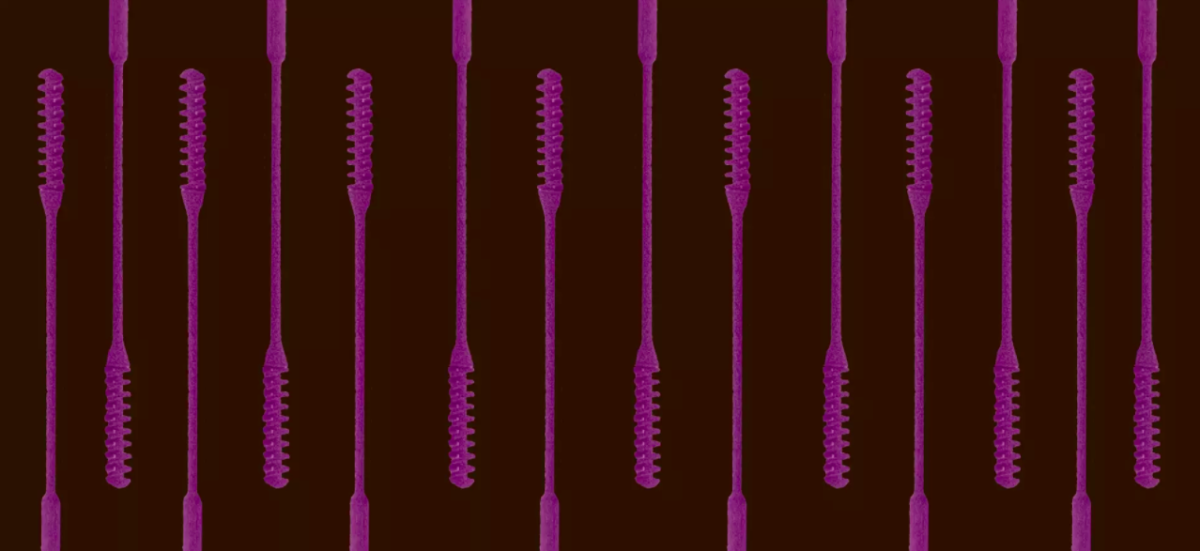
Another critical factor in the battle against coronavirus is widespread diagnostic testing. The most common tests use nasopharyngeal swabs to collect samples from the upper respiratory tract. However, these specially-designed swabs are currently in short supply, and commercially available cotton and wood swabs cannot be used to make an accurate diagnosis of SARS-CoV-2, the virus that causes COVID-19.
“The hospital is the battlefield, and the healthcare workers are the soldiers. Without the help of HP, we would not have enough weapons to beat this nightmare.” — Juan Angel Muñoz López, nursing supervisor, Príncipe de Asturias Hospital, Madrid, Spain
HP reacted swiftly to the nasal swab shortage. On March 19, Annunziata assigned a team to collaborate on 3D nasal swab designs and clinical trials with a multi-disciplinary team from Beth Israel Deaconess Medical Center (BIDMC), part of Beth Israel Lahey Health.
The effort is being coordinated by Annette Friskopp, global head and GM of HP Specialty Printing Systems, and Lihua Zhao, head of HP 3D Labs, within HP Labs in Palo Alto. Friskopp, who has led HP’s long-standing relationship with the BIDMC team through their use of the HP D300e Digital Dispenser, and Zhao quickly put an application R&D team based in San Diego into action and began working daily with Dr. Ramy Arnaout, associate director of the Clinical Microbiology Laboratories at BIDMC and faculty member of the Department of Systems Biology at the Harvard Medical School, to iterate and evaluate on swab designs. “Within 48 hours we had designed, printed, and shipped the prototypes for testing,” says Zhao.
The 3D-printed swab designed for HP Multi Jet Fusion is a nylon-based medical device that consists of a shaft, engineered with many features in one single-piece including a handle, a flexible neck, and a well-designed tip for efficacy in sample collection, and also for patient comfort and safety. Though it measures just 15 centimeters, “it actually incorporates so many different complexities, from the design to the performance to the production requirements — all packed into a little tiny swab,” says Zhao. “Fast iteration, fast prototyping, and decentralized manufacturing” makes 3D printing ideal for creating the products most urgently needed in local markets.
The HP-designed, 3D-printed nasal swab has been clinically validated by BIDMC and showed excellent concordance with the controls in the clinical trial. HP is now working with its global network of partners to plan for large-scale production to help address this critical shortage.
A decade of change in weeks
The 3D printing industry enables companies to produce customized parts on-demand — quickly and locally — reducing waste and eliminating the need for a far-flung manufacturer. The technology allows for the rapid design and production of crucial medical parts, which can be printed using digital files, and immediately get them into the hands of medical staff, which has been proven essential during the COVID-19 crisis.
Zhao notes that working on the nasal swab project has not only opened her mind to what 3D printing can do in the healthcare world but has also changed the mindset of those working in the medical field. “One doctor we worked with told me that after the crisis, 3D printing might change the way we do medicine — that equipment, devices, components, and instruments can be substituted and even improved using 3D printing.”
Annunziata agrees that the 3D printing landscape is changing rapidly in response to COVID-19. “We are taking risks but in a balanced way,” he says. “We can do things we never thought were possible, and more quickly than we ever thought possible.”
Learn more about how HP is supporting customers and communities during the global pandemic.